Navigational Bronchoscopy: A Biomedical Scientist’s Perspective
Andrea Davies, Consultant Biomedical Scientist, University Hospital of North Tees
In September 2019, the diagnostic cytology (DC) department at University Hospital of North Tees (UHNT) commenced a Rapid on-site Evaluation (ROSE) service to both the respiratory and gastrointestinal teams within North Tees & Hartlepool NHS Foundation Trust.
The respiratory ROSE requests are predominantly to support Navigational Peripheral Bronchoscopy and on occasion Endobronchial Ultrasound (EBUS). Both procedures are carried out in the clinical setting within either the endoscopy unit under sedation, or in theatre (the patient being subjected to general anaesthesia). Approximately 2-6 patients per week undergo these procedures at UHNT, with the clinical and radiological indications including diagnosis of suspected early cancer as well as mediastinal staging of thoracic cancers. The Navigational Peripheral Bronchoscopy service can also be accessed by patients outside of the region, when referred by a Respiratory consultant in charge of their care within the local Trust.
ROSE is requested for a variety of reasons, including but not limited to; previous undiagnostic procedures, high risk of bleeding due to the location of the target lesion or lymph node, small peripheral lung lesion that may prove difficult to access. ROSE provides a patient-centred service by facilitating specimen triage, ensuring adequate sampling/directing sampling, providing a rapid provisional diagnosis, reducing procedure times and avoiding unnecessary repeat examinations. ROSE supports the effective use of clinical and consultant time, helping to limit delays in diagnosis and treatment.
Navigational Peripheral Bronchoscopy:
Conventional flexible fiberoptic bronchoscopy has been routinely used for successfully diagnosing pulmonary lesions for many years. Despite revolutionizing respiratory diagnosis, a recognized limitation of the technique is low diagnostic yield from small peripheral lesions. The use of techniques such as Ultrathin bronchoscopy, paired with an ultraminiature radial endobronchial ultrasonography probe and virtual bronchoscopy navigation (VBN) for route planning are used in a complementary way as modified techniques developed to overcome this. The ultimate goal of these systems is to obtain material safely, using minimally invasive techniques.
VBN is a method in which a bronchial path is produced from virtual bronchoscopy images developed by a computer-aided software system, and used as a guide to navigate the bronchoscope to often tiny suspicious lesions in the peripheral bronchi. Reconstruction of pre-procedural CT images generates a 3D bronchoscopic view and path, which can be displayed digitally and used for reference prior to and during the procedure.
Adjunctive fluoroscopic imaging (portable C-arm X-ray machine) is used during the procedure to help guide the navigation (which requires all team members to wear protective aprons and thyroid guards). Any divergence from pre-procedural CT images can be addressed and sampling aided by confirming that the bronchoscope is in the desired location.
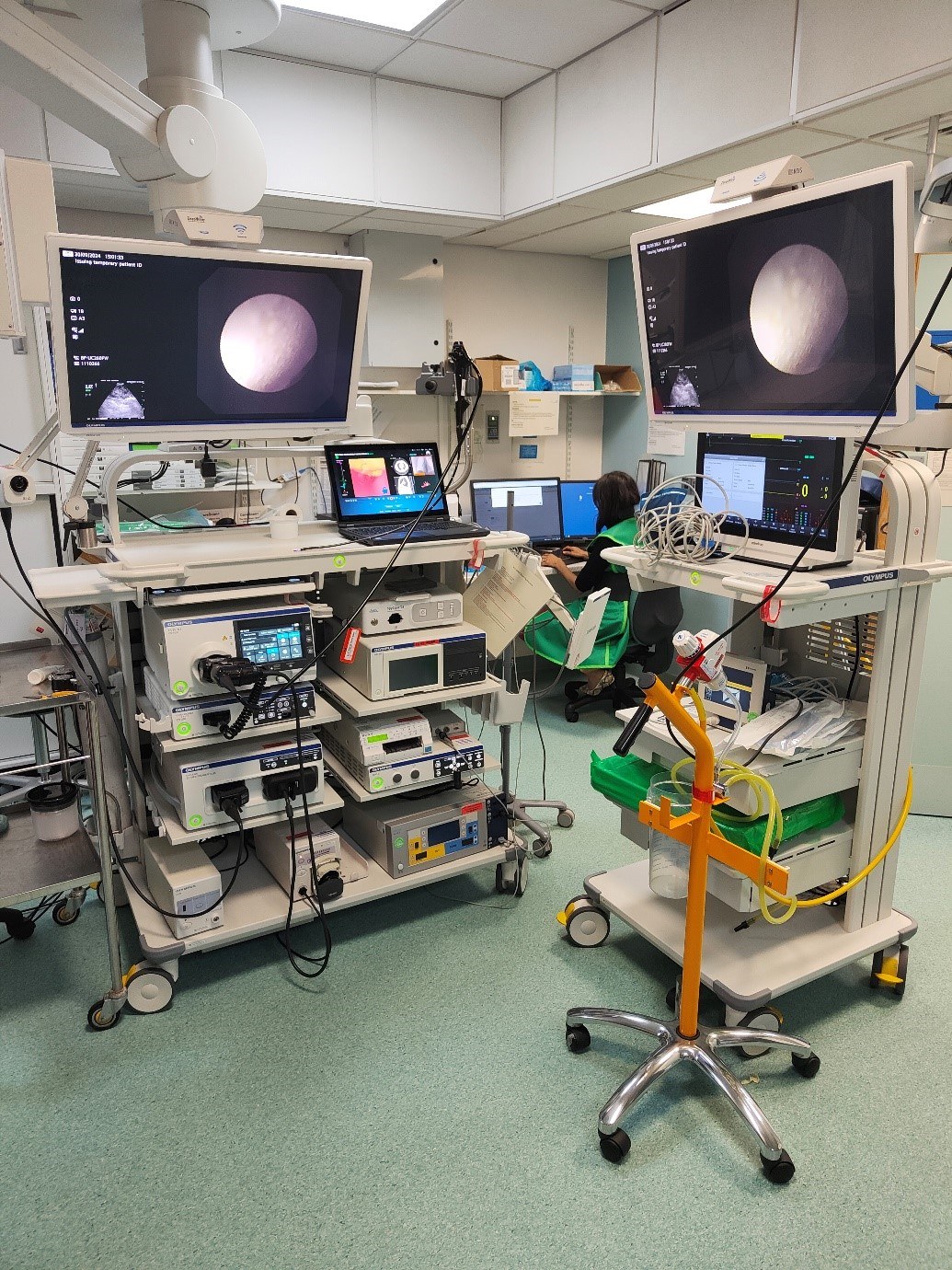
ROSE at UHNT:
Notification of the requirement for ROSE is e-mailed to the cytology department by a member of the lung health secretarial team at least one week ahead of the procedure date. The notification includes patient identification details, allowing for interrogation of the patients' health records prior to biomedical scientist attendance. Occasionally there are requests for attendance at procedures at relatively short notice, which are supported wherever possible.
Prior to the Navigational bronchoscopy or EBUS, the biomedical scientist has a discussion with the consultant to determine each patient's clinical context (which may not be fully determined from review of health records) and participates in the pre-procedure huddle. During the huddle each team member outlines their role, expected sampling methods are discussed, the process to follow in case of excessive bleeding is determined and the patient's medical history is presented, including co-morbidities.
Within the procedure room there should be sufficient space for specimen preparation, staining and microscopy; an independent light source may be required if overhead lighting is turned off for better visualisation of bronchoscopy and ultrasound images.

During the procedure, the attending biomedical scientist prepares air-dried slides for rapid Romanowsky staining from a range of specimens depending on the sampling methods employed, including; bronchial brushes, bronchial biopsies, cryobiopsies (larger pieces of tissue are obtained using a cryoprobe at temperatures well below freezing), fine needle biopsies (FNB) and fine needle aspirates (FNA).
Bronchial brushes are rolled over a clean labelled glass slide, prior to the brush head being placed into Hologic CytoLytTM collection medium; the aim being to transfer representative cells from all sides of the brush head onto the slide. Similarly, bronchial biopsies and/or cryobiopsies are removed from the forceps and placed onto a clean labelled glass slide and rolled across the surface using a 25 gauge needle, prior to being placed into formalin. For FNB/FNA samples a representative aliquot of the specimen is transferred (either drop wise or via a section of core) to the surface of a labelled glass slide and spread to distribute the material thinly for rapid air-drying, this can be facilitated by using a hand held fan. Bronchial alveolar lavages are not routinely assessed by ROSE.
Once stained and dry, each slide is assessed microscopically for the presence of diagnostic cells, specimen adequacy, and cellularity for subsequent ancillary testing. The information obtained by the biomedical scientist from the slide assessment is relayed to the consultant, in a timely manner, using sensitive language appropriate to the situation, i.e. when the patient is under mild sedation versus general anaesthesia.
Ultimately, the decision to end the procedure lies with the bronchoscopist based upon a number of factors including bronchoscopic impression, ease of sampling, patient tolerance and safety; however, in most situations this decision is largely influenced by ROSE findings.
Once all of the specimens have been processed by the cellular pathology laboratory the whole case is assessed and a final report issued by a Consultant Pathologist. A database is maintained of all ROSE cases attended and periodically audited for final patient outcomes.
ROSE at UHNT is currently provided by two part-time dedicated diagnostic cytology staff and to date we have attended approximately 450 patient procedures; 400 respiratory and 50 gastrointestinal. Despite extensive cytology screening experience, the initial setting up of this arm of the cytology department proved to be a steep learning curve to us as Senior Biomedical Scientists. From its introduction in 2019 to the present, there have been a number of challenges and changes to the service, for example performing navigational peripheral bronchoscopy procedures in theatres with the patient under general anaesthesia; we continue to respond to such developments in a positive manner, continually improving how we deliver ROSE.
During bronchoscopy or endoscopic procedures the role of the biomedical scientist can vary widely from advising on specimen acquisition to confirming lesional sampling, indicating the presence of malignant cells and occasionally directing the route/location for optimal sampling. This requires the biomedical scientist to possess a diverse skill set in addition to pathological, clinical and radiological knowledge, including confidence, empathy, resilience, being resourceful, possessing excellent communication skills and fine motor skills (particularly when handling tiny biopsy specimens), alongside efficient microscopy and screening skills.
Quality assurance:
ROSE clinic biomedical scientist impressions versus cytology final reports and/or histology outcomes, are continually audited and show a consistently high level of concordance. Winning the Northern Cancer Alliance Team Award for diagnosing early stage lung cancers in October 2024, served as just recognition of the dedication and hard work of the whole Navigational Bronchoscopy team at UHNT.

