What can digital cytology offer the laboratories and the UK screening programmes?
Professor Allan Wilson Consultant Biomedical Scientist and Laboratory Manager at Monklands Hospital and Clinical Lead for the Scottish Cervical Screening Programme.

I have been using the Hologic genius system routinely for more than two years and have assessed over 5000 cases in the system. I have recorded my opinions for both the Genius system and standard microscopy for analysis. I have also recorded the time taken to report on both systems for around 1200 cases. Based on my experience to date, there is no doubt that digital cytology holds significant potential to improve the laboratory service across the UK.
There is currently a study involving two labs in England to compare results from cases reported using standard microscopy and the Genius system. The two labs are working through a protocol to introduce the imager stain and to gather the data to compare the two assessment systems.
The Scottish cervical screening programme (SCSP) has been using the Hologic Image Assisted Screening (IAS) system for more than ten years and have demonstrated a reduction in false negative reporting and increased sensitivity and productivity. The Hologic Genius system uses a similar algorithm to the IAS but with the obvious difference is that the cytologist assesses the digital slide image rather than the glass slide. The principle is the same: the algorithm identifies the areas where abnormal cells are most likely to be present and presents them to the cytologist to interpret. If the areas identified do not contain any abnormal cells the sample can be signed out as negative without an assessment of the full slide.
The new Digital Imager significantly reduces the need for a microscope as it presents the cells which are most likely to be abnormal as digitised images. Glass slide review will still be required for difficult cases but this will only be for a very small percentage of cases. Microscopes will not be required for all staff. The Scanner has a capacity of 400 slides with continuous operation and loading. The objects of interest are presented on the digital Review Station in a main gallery of 30 tiles. An additional 30 tiles (5 rows of 6 objects) are available, by clicking the drop-down arrow beside each row if the reviewer requires additional information to make the diagnosis. The gallery is presented on the left side of the screen with the accompanying full cell circle on the right side of the screen. By clicking any object in the gallery, the object is presented at 40x magnification within the cell spot for the reviewer to view in context.

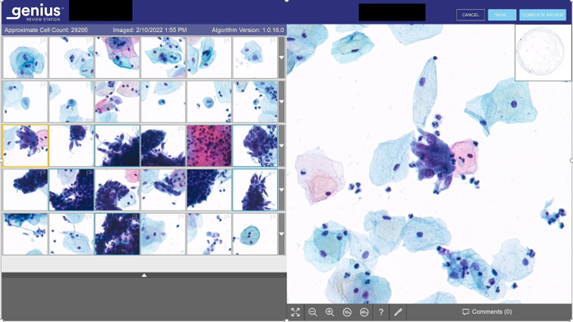
The tiles will always show cells. 30 into 60 tiles:
- 1st Row: “large cells” with low NCR including intermediate cells and low grade dyskaryosis
- 2nd Row: “small single cells” with high NCR including parabasals and high grade dyskaryosis
- 3rd Row: “bizarre forms, spindle cells”, metaplastic cells
- 4th Row: “Glandular cells” - endocervical, endometrial cells and hyperchromatic crowded groups
- 5th Row: “Infections/pseudo infections”
Use of digital technology and artificial intelligence in other screening programmes
The use of digital systems and Artificial Intelligence (AI) is currently being assessed in the breast screening programme where an increasing evidence base is emerging on the increased sensitivity and productivity of digital systems and it is likely that digital technology and AI will be approved for use in the programme within a few years.
Similar technology is being assessed in the diabetic retinopathy screening programme with promising results. The common theme is that suitably “trained” digital systems using AI are better than humans in identifying significant rare events in complex images.
Digital histopathology is now the routine reporting system in the many labs in the UK and the use of algorithms to suggest reports is now firmly on the agenda particularly in dermatopathology. There are obvious advantages in harnessing this technology to improve the cervical screening programme in terms of effectiveness and efficiency.
What about internal quality control?
Although there is no obvious “rapid” method of using the Genius system for IQC to replicate the current rapid preview or review, the arrangement of the tiles in stratified rows. provides a relatively easy identification of abnormal cells in the top two rows. This approach could easily be introduced to replace the current rapid preview/review and provide a digital equivalent to the current slide based IQC procedures.
Advantages of digital cytology
The emerging data from Germany, Belgium and other northern mainland European countries where this technology is already being used diagnostically is that it is superior to both manual microscopy and the Hologic IAS system. The gains are in terms of both effectiveness, the Genius system detects more high grade disease and efficiency, the system is significantly “quicker” at all stages of assessment.
Digital cytology has significant potential in training, particularly for medical staff where the programme has struggled to engage with trainee pathologists across the country since the roll out of HPV primary screening. A digital cytology training programme will lower the barrier of being attached to a cytology lab. Training for all staff will also be significantly easier using digitised images which can be easily shared across the country.
The use of digital cytology loosens the physical connection to the lab and will allow reporting from any site with the technology and the IT infrastructure. This has the potential to facilitate a fluid backlog management system to allow labs with backlogs at any stage of the sample pathway to transfer cases to another lab.
The technology may also mitigate the risk of future staff loss due to further consolidation of cytology labs. Staff will no longer need to attend the lab every day and can report remotely from home or from another lab. The current age profile of cytologists across the UK is a risk to service delivery and staff considering retirement may consider remaining in service if remote working using digital cytology is an option.
Limitations and challenges
Like many digital systems, the scanners struggle with hyperchromatic crowded groups where careful focussing is required to interpret this challenging presentation. These cases will often require microscopic assessment to ensure correct interpretation.
National guidance is required for storage of digitised slides, especially as they may form part of the invasive cancer audit in the future.
Next steps
The data on the Scottish trial needs to be analysed and potentially used to validate the technology or form the basis of a peer-reviewed paper. The challenge is finding the time and the resource to analyse the data while trying to run a busy screening laboratory
The study in the two labs in England is a slightly different approach and may well be completed before data emerges from the Scottish study. The obvious next step is to submit data to the national Screening Committee (NSC).
Like any digital installation, success is entirely dependent on a robust IT infrastructure within local networks and the ability to work across traditional NHS network boundaries. Laboratories should engage with local IT teams well in advance of installation of new technology.
The future?
There is no doubt that digital cytology is the way forward for cervical cytology. Lessons should be learned from the transition to digital histopathology and servers and monitors can be shared.
There is a potential for using digital cytology systems such as the Genius technology to assess non-gynae samples. Respiratory and urine cytology would seem to be the obvious starting point but algorithms will no doubt emerge for fluids and FNA preparations.
The next logical step, to be slightly controversial is to suggest that the next development in digital cytology is a system that could state:
“based on the assessment of the digitised image for this case, a suggested report is “High grade dyskaryosis is present” please confirm if you agree”
The UK screening programmes do not have a strong history of introducing new technology quickly but there is genuine pressure on all labs in the UK and we cannot delay in assessing this new technology safely but quickly to reduce the risk to service delivery.
